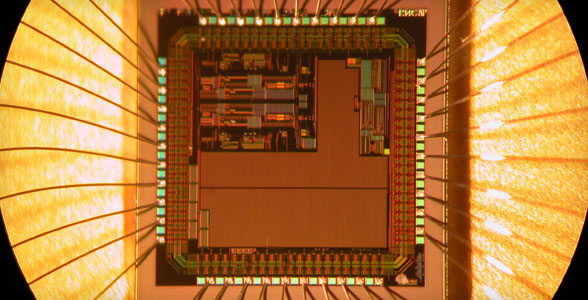
For diabetics, knowing the exact amount of glucose in the bloodstream is crucial. But the routine of drawing blood, testing glucose levels, and injecting insulin means the diabetic is tethered to a variety of intrusive devices. Beyond the discomfort and inconvenience caused by testing, maintenance of a stable blood glucose concentration is difficult to achieve by periodic insulin injection.
Steven Ripp and James Fleming, senior researchers at UT’s Center for Environmental Biotechnology (CEB), hope to eliminate the need for needles and test kits by developing a small, implantable device that can detect glucose in the bloodstream and activate release of insulin only when needed by the body. CEB is a collaborative research partner of UT’s Institute for a Secure and Sustainable Environment (ISSE).
Ripp and Fleming’s device builds on the technology behind the bioluminescent bioreporter integrated circuit (BBIC), which layers living organisms atop a microchip about half the size of the nail on your pinkie. The organism is a bioluminescent reporter, a genetically engineered single-celled organism that responds to the presence of a target substance—in this case, glucose—by emitting light. The intensity of the light reflects the quantity of the target substance. In a diabetic, bright or high-intensity light would signal rising glucose levels and trigger an implanted pump to release insulin.
The applications for this new technology extend well beyond glucose monitoring in diabetics. BBICs could protect human and environmental health in almost limitless ways.
Ripp and Fleming are working on a National Science Foundation project to develop a device to monitor and control thyroid hormone levels in the body. “Imbalanced thyroid hormone contributes to a wide variety of physiological and psychological conditions,” Fleming says.
Detecting common food-borne contaminants is also a chief area of interest. According to the Centers for Disease Control and Prevention, about 76 million Americans suffer food-borne illnesses each year. Of those, 300,000 are hospitalized and 5,000 die.
A BBIC could be tossed into the water used to rinse fruits and vegetables to detect the presence of the pathogens Escherichia coli (E. coli) or Salmonella. BBICs engineered to detect a range of food-borne toxins—including histamine, a common contaminant in seafood—could be used in food packaging. Toxins and pathogens present in foods could cause a BBIC-containing dot on the package to change color or transmit a radio–frequency signal to a hand-held receiver, allowing grocers to identify and discard spoiled food.
Masks and respirators equipped with BBICs could detect threats carried on the air. Likewise, devices placed in intake ducts in subway stations, office buildings, homes, or even submarines—any enclosed space, says Ripp—could monitor for airborne contaminants.
Ripp and Fleming are at work on a “smart” bandage that would both treat infection and identify its source. Bacteriophages are naturally occurring viruses that feed entirely on bacteria. Ripp and Fleming are genetically engineering bacteriophages to emit light in the presence of targeted bacterial pathogens—in this case common disease agents like strep, staph, and Pseudomonas—that could infect an open wound.
A cotton bandage infused with specially engineered bacteriophages and outfitted with a BBIC could prevent or treat infection and monitor the condition of soldiers with minor wounds who have returned to the battlefield.
“With major wounds, as soldiers are being transported to triage units, the chips would send out a signal alerting medical personnel to a source of infection so they can be ready to treat it,” says Ripp.
With funding from the National Institutes of Health, Ripp and Fleming are also developing sensors to detect the signatures produced by cancer cells, which could enhance doctors’ ability to detect and treat the disease in its earliest stages.
Alice Layton, a CEB research associate professor and a colleague of both Ripp and Fleming, is pursuing another avenue of biotechnological sleuthing, in this case to trace the source of contaminants entering rivers and streams.
Stock Creek, which flows into the Tennessee River near Knoxville, has been designated by the U.S. Environmental Protection Agency as a 303(d) stream because of contamination by E. coli bacteria commonly found in fecal matter. But where does the bacteria come from? Humans? Livestock? Wildlife? Stock Creek is located in a rural environment where farm animals are present, and a number of failed or failing septic systems nearby could also introduce human waste into the stream.
Because of the bacteria’s wide range and ability to propagate outside the digestive system, E. coli alone cannot be used to determine whether the fecal contamination is from humans, livestock, or wildlife. Without a clear indication of the source, resource managers may have a difficult time controlling the contamination.
Layton found a way around the problem by developing assays to target Bacteroides, a genus of bacteria that is abundant in the digestive systems of all mammals but whose individual species are host specific, so they can be used to determine the animal of origin.
An initial visual inspection of the watershed helps Layton and her colleagues identify likely sources of the contamination. “Based on visual observations, you can say that the most likely sources are humans, because there are many houses with septic systems, or that the suspected source of contamination may be livestock if there are a lot of cattle in the area.”
Once she has narrowed the number of potential sources, Layton runs the assay on water samples from the creek and looks for the distinctive genetic signature of the prime suspects.
“This technique is highly reliable because of its ability to distinguish between human, bovine, and total Bacteroides in pinpointing the source,” Layton says. “You can’t do that with E. coli.”
Having developed the technique in the CEB laboratory, Layton and her colleagues took the research to the creek. With support from the Tennessee Department of Environment and Conservation, Layton worked with an interdisciplinary team that included Larry McKay, Jones Professor of Hydrogeology in UT Knoxville’s Department of Earth and Planetary Sciences, and Randall Gentry, associate professor in UT Knoxville’s Department of Civil and Environmental Engineering and director of ISSE.
Using Layton’s assays and combining stream sampling in the field with groundwater hydrology and statistical modeling gives a clearer picture of the watershed and a better determination of the source of fecal contamination—which was found to come from both cattle and human sources. Test results, while confirming common-sense suspicions, provide precise information that can be used as a baseline as efforts are made to reduce both types of contamination.
“State regulators are excited to have this tool available for working in bacterially contaminated streams and coming up with a plan to delist them,” says Gentry. Such plans might include implementing best management practices to exclude cattle from the creek or replacing failing septic tanks.
Since identifying the key sources of Stock Creek’s E. coli contamination, Layton and her colleagues have applied the technology on other E. coli contaminated waterways in Tennessee, as well as to New York’s Hudson River and streams in Bangladesh.–Marshall Brown, Elise LeQuire, and David Brill