By Jackie Wise | Photos by Yasmin Murphy
“I’m going to look back at this and think we helped with that, we helped create a world that’s a better, safer place for our children,” says Jennifer Winbigler.
A passion for their community and a drive to create a safe environment for their three children led Brian (Knoxville ’03, Health Science Center ’14) and Jennifer (Knoxville ’06, Health Science Center ’13) Winbigler past the front lines of the COVID-19 pandemic as they built solutions on a local level and helped break ground on hope worldwide.
Outside of their full-time jobs, Brian, an assistant professor in the UT Health Science Center Department of Clinical Pharmacy and Translational Science, and Jennifer, a hospitalist at Blount Memorial Hospital, run Winbigler Medical, where they provide general internal medicine services and on-site clinics in Maryville.
COVID-19 intertwined their personal and professional lives in a way that hadn’t existed before, and as schools opened back up, the Winbiglers saw a need in their local district. If teachers developed symptoms of COVID-19, they had to remain home until they could produce a negative test result. Those results could take a week or more, and teachers out of the classroom were leading to children out of the classroom.
“It’s the kids who don’t have that support at home that are going to suffer, that have suffered, and it’s going to create an even bigger social disparity,” Jennifer says on the drive to help keep Maryville schools from going virtual.
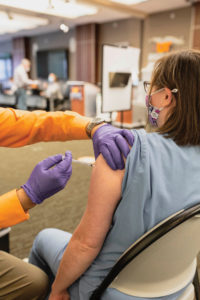
Using their own money, Brian and Jennifer purchased $5,000 worth of COVID-19 diagnostic tests and, through a relationship with a local lab, provided a 24-hour turnaround on results at no charge to teachers. Before long, other essential workers reached out, and they went from helping maintain safety within the schools to safety throughout the community, performing tests on a daily basis.
Through their professional and personal lenses, they were seeing the effects of COVID-19 that went beyond the virus. They saw the strain on the health-care system, the influx of patients and the difficulty in treating everyone quickly and efficiently. They watched their children deal with the changes in school and social routines and were all in when it came time to fight COVID-19.
Pre-virus, Jennifer spent a few days a month working as a sub-investigator on clinical trials with the Alliance for Multispecialty Research/New Orleans Center for Clinical Research. It was there that she and Brian had the opportunity to take part in the trials of four vaccines, and they said the experience was unlike anything they had seen before.
Prior to the COVID-19 vaccines, the fastest vaccine developed was the Mumpsvax, which took four years and was licensed in 1967. Since that time, technology has grown considerably, and scientists sequenced the entire coronavirus genome in less than a month. Brian, who managed the pharmacy throughout the trials, explained that knowing what parts of the virus that bodies recognize as foreign, and being able to sequence those parts in a laboratory setting, put the coronavirus vaccine development quickly ahead of anything else in history.
Also making history was the number of volunteers participating in the vaccine trials. The Winbiglers had people coming to them in the hopes of receiving the vaccine so they could hug their grandchildren again, go to their jobs protected and advance medicine to help end the pandemic.
“We didn’t have trouble enrolling. People were coming to us, which in most trials we have to actively recruit, advertise and do all those things,” says Brian.
Each phase of a clinical trial serves a different purpose in the study of safety, efficacy, dosing and adverse reactions, but the full process can take years. For COVID-19 vaccine development, researchers were able to run phases one, two and three concurrently, while maintaining the normal data collection and safety standards required.
This change to the clinical trial process allowed manufacturers to evaluate vaccine effectiveness and generate scientific data to apply for an Emergency Use Authorization through the Food and Drug Administration. While the timeline seems fast, each vaccine is rigorously tested before approval.
Regarding the long-term side effects of the vaccines, Jennifer says, “We don’t know the long-term effects of COVID, and honestly, the long-term effects of COVID scare me more than the long-term effects of a well-studied, well-investigated vaccine.”
“If anything, the vaccine—if you get it and you should get COVID, it’s lowering your risk of any complications and that main one being death,” says Brian.
In addition to the reduction in severe outcomes, the vaccine also helps contain the virus from mutating into new variants. These variants can cause milder or more severe illness, alter the effectiveness of current treatments or change how the virus is spread.
“By reducing spread, you reduce the opportunity for the virus to mutate and change because, the fewer people that it’s exposed to, the less likely it is to adapt,” Brian explains.
From helping maintain normalcy for children in their classrooms to finding ways to fight COVID-19 through vaccinations, the Winbiglers are hopeful that the work they have done, and are still doing, will create a better and safer world.
“As a physician, I can only help those that I can physically touch, usually, but doing this I feel like I can touch the entire world and help heal the entire world by doing the work that I did,” says Jennifer.